Europe Biosimilar Market Outlook
Base Year: 2024
Historical Years: 2019-2024
Forecast Years: 2025-2033
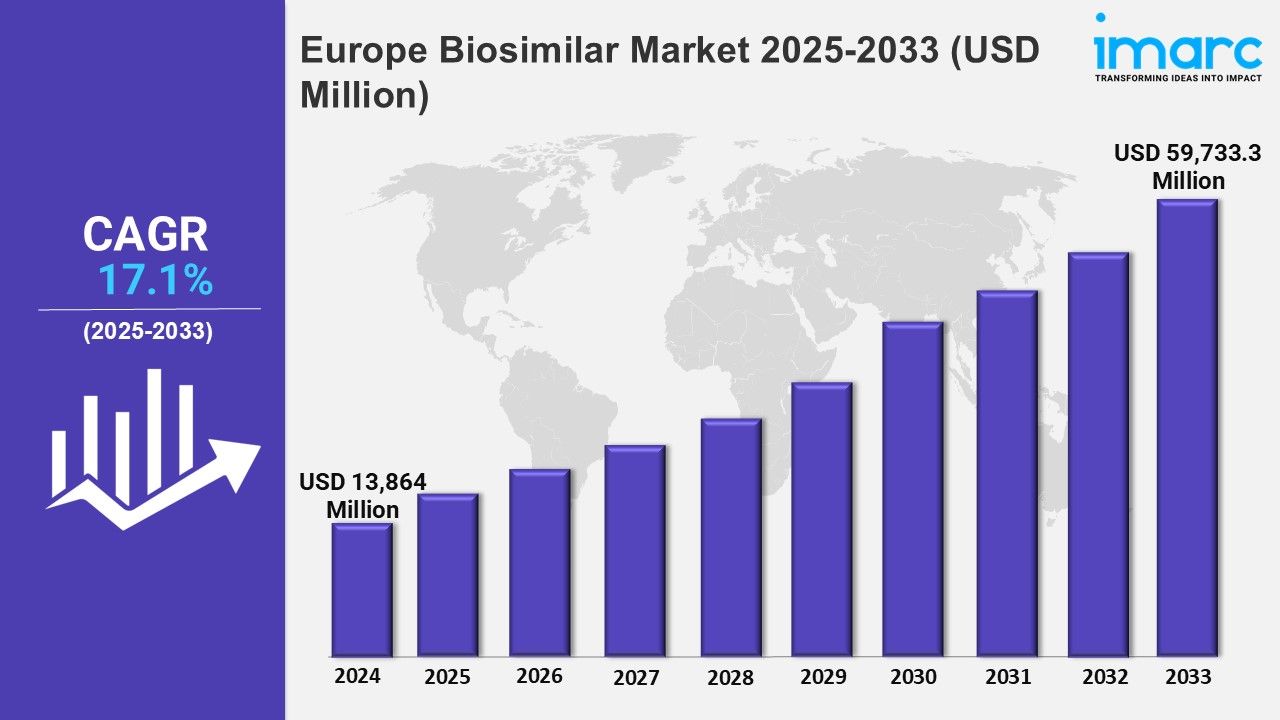
Market Size in 2024: USD 13,864 Million
Market Forecast in 2033: USD 59,733.3 Million
Market Growth Rate: 17.1% (2025-2033)
The Europe biosimilar market was valued at USD 13,864 Million in 2024 and is projected to grow to USD 59,733.3 Million by 2033, with an expected compound annual growth rate (CAGR) of 17.1% from 2025 to 2033.
For an in-depth analysis, you can refer free sample copy of the report: https://www.imarcgroup.com/europe-biosimilar-market/requestsample
Europe Biosimilar Market Trends:
The expanding market within Europe is chiefly propelled by the increasing demand for cost-effective treatment alternatives, especially among chronic disease patients. In this regard, the rising prevalence of biologic drugs nearing patent expiration helps in the greater access of the biosimilars, thus aiding their market growth. In addition, improved healthcare infrastructure within the area is aiding the adoption of biosimilars, which is supported by a favorable regulatory environment. Furthermore, the growing expenditure on healthcare in Europe empowers both patients and providers to consider biosimilars as real alternatives.
Also, the rise in the urgent need to cut down on the overall expenditure of healthcare has stimulated the consumption of biosimilars as they also confer huge cost savings without any compromise on the intended effect of the treatment. In addition, the growing support of government bodies and health organizations for the development of biosimilars constitutes a significant growth-inducing factor. Also, increased awareness campaigns on the safety and efficacy of biosimilars are promoting the acceptance of these products among healthcare professionals and patients. Investments by pharmaceutical companies in biosimilar R&D are further market-enhancing behaviors that shape innovation and competition.
Europe Biosimilar Market Scope and Growth Analysis:
The market is being broadly expanded by the growing number of therapeutic applications in the fields of oncology, immunology, and endocrinology, which are addressing existing clinical needs. In addition, the increasing adoption of biosimilars into hospital settings is aiding their acceptance into the main treatment protocols, thus reinforcing the market stance. Also, the increasing availability of cheap biosimilar drugs is providing equitable access to advanced therapies, particularly in emerging economies across Europe. Also, strong collaborations between the regulators and the pharmaceutical companies help in establishing a fast-growing approval process for products and their launches.
This has also been enhanced by the presence of local manufacturers catering to region-specific demands and preferences. Adoption of advanced manufacturing technologies is improving production efficiency, cost reduction, and supply chain dynamics. Furthermore, increased focus on biosimilar interchangeability will foster confidence in prescribers, thereby enhancing market acceptance. According to the market analysis, current reimbursement policies favouring biosimilar adoption are changing the outlook of the market by making it conducive for both the players of industry and health systems.
Ask Analyst For Customization: https://www.imarcgroup.com/request?type=report&id=1023&flag=C
By the IMARC Group, the Top Competitive Landscapes Operating in the Industry:
- Novartis
- Pfizer
- Teva
- Celltrion
- Merck Sharp & Dohme
- Samsung Bioepis
- Eli Lilly
- Accord Healthcare Ltd.
- Amgen
- Boehringer Ingelheim
- Hexal Ag
- Apotex
- Stada Arzneimittel Ag
- Ratiopharm
- Mylan
Europe Biosimilar Industry Segmentation:
The market report offers a comprehensive analysis of the segments, highlighting those with the largest Europe biosimilar market share. It includes forecasts for the period 2025-2033 and historical data from 2019-2024 for the following segments.
Breakup by Molecule:
- Infliximab
- Insulin Glargine
- Epoetin Alfa
- Etanercept
- Filgrastim
- Somatropin
- Rituximab
- Follitropin Alfa
- Adalimumab
Breakup by Indication:
- Auto-Immune Diseases
- Blood Disorder
- Diabetes
- Oncology
- Growth Deficiency
- Female Infertility
Breakup by Manufacturing Type:
- In-house Manufacturing
- Contract Manufacturing
Breakup by Country:
- Italy
- Germany
- United Kingdom
- France
- Spain
- Rest of Europe
Key highlights of the Report:
- Market Performance (2019-2024)
- Market Outlook (2025-2033)
- COVID-19 Impact on the Market
- Porter’s Five Forces Analysis
- Strategic Recommendations
- Historical, Current and Future Market Trends
- Market Drivers and Success Factors
- SWOT Analysis
- Structure of the Market
- Value Chain Analysis
- Comprehensive Mapping of the Competitive Landscape
Note: If you need specific information that is not currently within the scope of the report, we can provide it to you as a part of the customization.
About Us:
IMARC Group is a global management consulting firm that helps the world’s most ambitious changemakers to create a lasting impact. The company provide a comprehensive suite of market entry and expansion services.
IMARC offerings include thorough market assessment, feasibility studies, company incorporation assistance, factory setup support, regulatory approvals and licensing navigation, branding, marketing and sales strategies, competitive landscape and benchmarking analyses, pricing and cost research, and procurement research.
Contact Us:
IMARC Group
134 N 4th St. Brooklyn, NY 11249, USA
Email: [email protected]
Tel No:(D) +91 120 433 0800
United States: +1-631-791-1145